Take on ATTR-CM at the source
The first and only silencer for ATTR-CM
AMVUTTRA® (vutrisiran) works with your body to reduce the production of TTR in the liver, where most of it is made.


RAPIDLY KNOCKS DOWN TTR
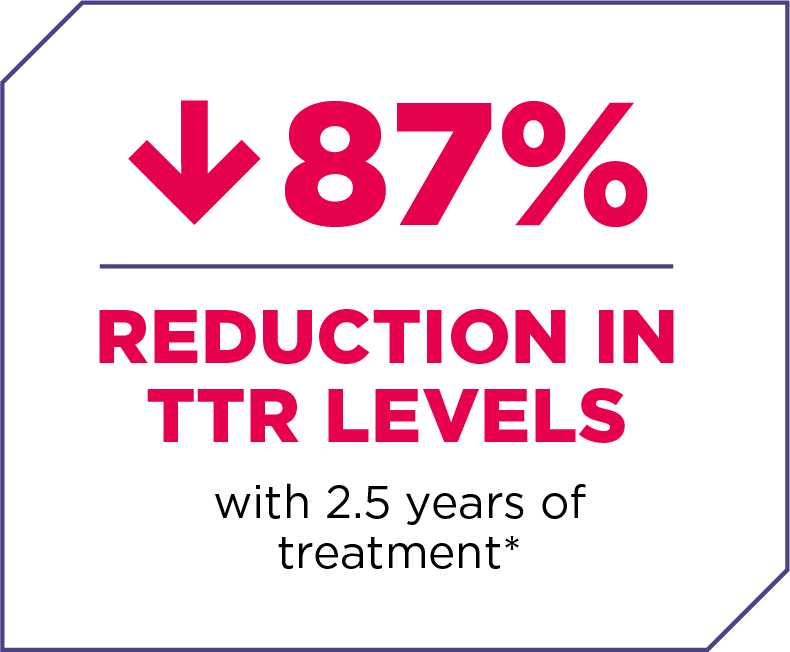
AMVUTTRA® (vutrisiran) works with your body to reduce the production of TTR in the liver, where most of it is made.



Treatment with AMVUTTRA lowers the amount of vitamin A in your blood. Your doctor will tell you to take a vitamin A supplement every day. You should not take more than the amount of vitamin A recommended by your doctor.
Low vitamin A levels can affect vision. If you have problems with your vision (e.g., night blindness) while taking AMVUTTRA, talk to your doctor. Your doctor may refer you to an eye specialist.
The most common side effects of AMVUTTRA were pain in the arms or legs, pain in the joints, shortness of breath, and low vitamin A levels.
These are not all the possible side effects of AMVUTTRA. Talk to your doctor about side effects that you experience. You are encouraged to report negative side effects of prescription drugs to the U.S. Food and Drug Administration (FDA). Visit www.fda.gov/medwatch, or call 1‑800‑FDA‑1088.
For additional information about AMVUTTRA, please see the full Prescribing Information.
AMVUTTRA is a prescription medicine that treats the:
For additional information about AMVUTTRA, please see the full Prescribing Information.
Treatment with AMVUTTRA lowers the amount of vitamin A in your blood. Your doctor will tell you to take a vitamin A supplement every day. You should not take more than the amount of vitamin A recommended by your doctor.
Low vitamin A levels can affect vision. If you have problems with your vision (e.g., night blindness) while taking AMVUTTRA, talk to your doctor. Your doctor may refer you to an eye specialist.
The most common side effects of AMVUTTRA were pain in the arms or legs, pain in the joints, shortness of breath, and low vitamin A levels.
These are not all the possible side effects of AMVUTTRA. Talk to your doctor about side effects that you experience. You are encouraged to report negative side effects of prescription drugs to the U.S. Food and Drug Administration (FDA). Visit www.fda.gov/medwatch, or call 1‑800‑FDA‑1088.
For additional information about AMVUTTRA, please see the full Prescribing Information.
AMVUTTRA is a prescription medicine that treats the:
For additional information about AMVUTTRA, please see the full Prescribing Information.