PROVEN TO HELP PEOPLE WITH ATTR-CM
People receiving AMVUTTRA® (vutrisiran) lived longer and had fewer hospital visits

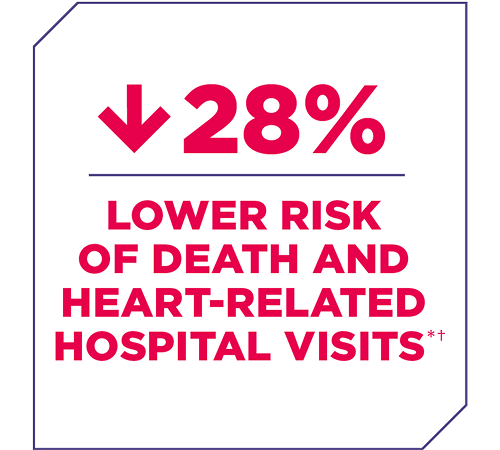
Effectiveness was determined by comparing the results of people treated with AMVUTTRA to those who received placebo (a treatment without any medication). The main measure of effectiveness was the combined risk of death and heart-related hospital stays and urgent visits over 3 years.


After the first 3 years of the study, people receiving placebo switched to AMVUTTRA so that everyone still in the study received AMVUTTRA for up to an additional 6 months. The study then measured the risk of death over 3.5 years, comparing the original AMVUTTRA group to the original placebo group.
†Most deaths were heart-related.
AMVUTTRA slowed disease progression compared to placebo‡
‡Patients receiving AMVUTTRA and patients receiving placebo walked a shorter distance and had lower KCCQ overall summary scores than at the start of the study.


