The site you are about to enter is intended for US healthcare professionals.
Are you a US healthcare professional?
Confirm and enter Visit patient siteAMVUTTRA® (vutrisiran) significantly improved nerve function
and quality of life for people at 9 months
People taking AMVUTTRA saw continued improvement over 18 months compared to people taking placebo in a similar study.
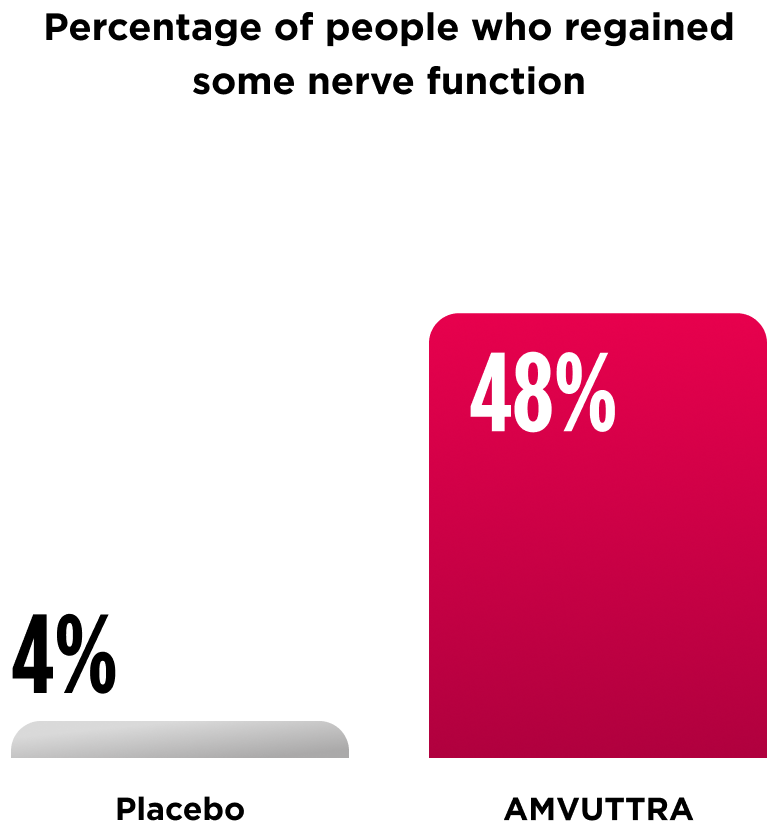
A separate analysis looked at nerve function at 18 months.
Almost half of people who received AMVUTTRA regained some nerve function at 18 months. Those who didn't regain some nerve function saw slower progression of their nerve symptoms compared to placebo.

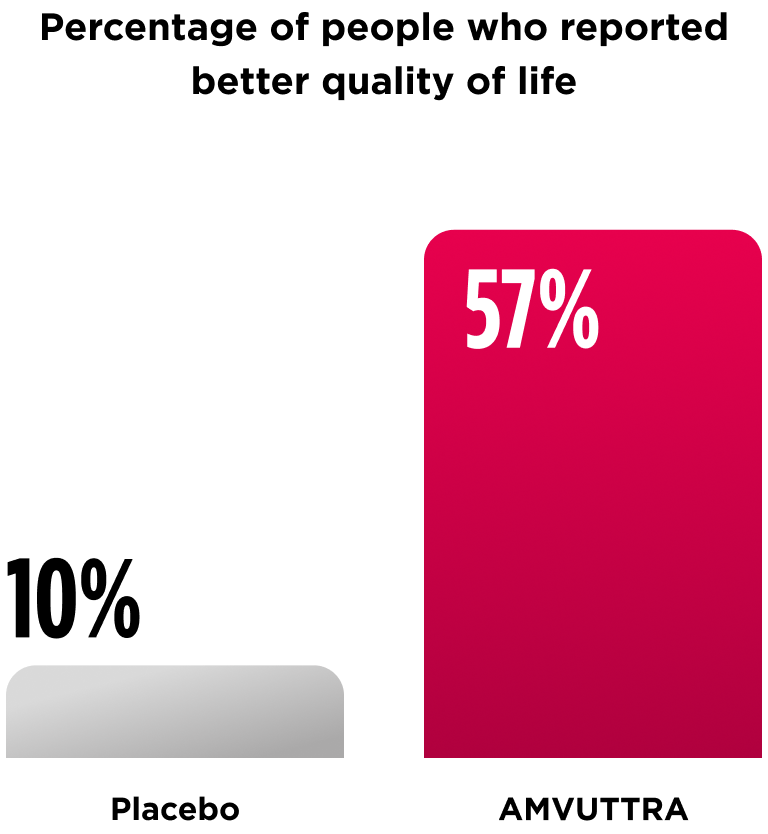
A separate analysis looked at quality of life at 18 months.

People saw other positive effects in their daily life
At 18 months, people taking AMVUTTRA did better than those on placebo in the following ways:

Maintained better walking speed
Walking speed was evaluated using the 10-meter walk test (10-MWT), a stopwatch-timed measure of a person’s walking speed over 10 meters, which is an indication of their mobility and balance.

Improved nutritional health
Nutritional health was evaluated using modified body mass index (mBMI), an assessment of height, weight, and the balance of fluids in the body, as well as a measure of nutritional status and heart health.

Performed daily activities more easily
Daily activities were evaluated using the Rasch-built Overall Disability Scale (R-ODS) questionnaire that assesses the ability to participate in common daily activities such as showering, walking up a flight of stairs, and dressing the upper body.
What are the most important things I should know about AMVUTTRA® (vutrisiran)?
AMVUTTRA can cause low vitamin A levels
Treatment with AMVUTTRA lowers the amount of vitamin A in your blood. Your doctor will tell you to take a vitamin A supplement every day. You should not take more than the amount of vitamin A recommended by your doctor.
Low vitamin A levels can affect vision. If you have problems with your vision (e.g., night blindness) while taking AMVUTTRA, talk to your doctor. Your doctor may refer you to an eye specialist.
What are the common side effects of AMVUTTRA?
The most common side effects of AMVUTTRA were pain in the arms or legs, pain in the joints, shortness of breath, and low vitamin A levels.
These are not all the possible side effects of AMVUTTRA. Talk to your doctor about side effects that you experience. You are encouraged to report negative side effects of prescription drugs to the U.S. Food and Drug Administration (FDA). Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
For additional information about AMVUTTRA, please see the full Prescribing Information.
What is AMVUTTRA?
AMVUTTRA is a prescription medicine that treats the:
Important Safety Information and Indications
What are the most important things I should know about AMVUTTRA® (vutrisiran)?
AMVUTTRA can cause low vitamin A levels
Treatment with AMVUTTRA lowers the amount of vitamin A in your blood. Your doctor will tell you to take a vitamin A supplement every day. You should not take more than the amount of vitamin A recommended by your doctor.
Low vitamin A levels can affect vision. If you have problems with your vision (e.g., night blindness) while taking AMVUTTRA, talk to your doctor. Your doctor may refer you to an eye specialist.
What are the common side effects of AMVUTTRA?
The most common side effects of AMVUTTRA were pain in the arms or legs, pain in the joints, shortness of breath, and low vitamin A levels.
These are not all the possible side effects of AMVUTTRA. Talk to your doctor about side effects that you experience. You are encouraged to report negative side effects of prescription drugs to the U.S. Food and Drug Administration (FDA). Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
For additional information about AMVUTTRA, please see the full Prescribing Information.
What is AMVUTTRA?
AMVUTTRA is a prescription medicine that treats the:
cardiomyopathy of
ATTR amyloidosis
polyneuropathy of hereditary
ATTR amyloidosis
transthyretin
transthyretin-mediated
amyloidosis
Kansas City Cardiomyopathy
Questionnaire
6-minute walk test
modified Neuropathy
Impairment Score +7
Quality of Life-Diabetic
Neuropathy