The site you are about to enter is intended for US healthcare professionals.
Are you a US healthcare professional?
Confirm and enter Visit patient siteSafety and effectiveness of AMVUTTRA® (vutrisiran) were shown in 2 clinical studies
The ATTR-CM study
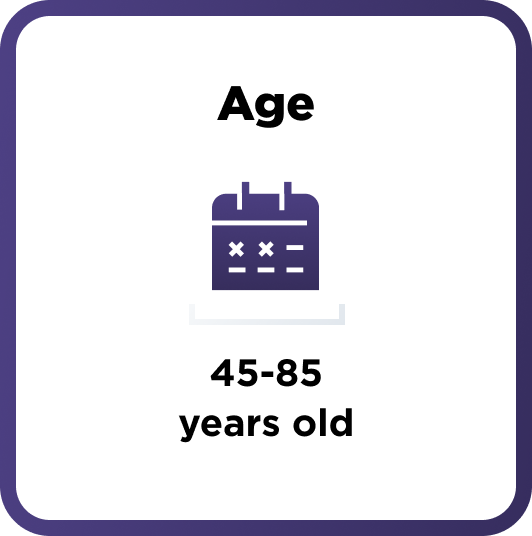
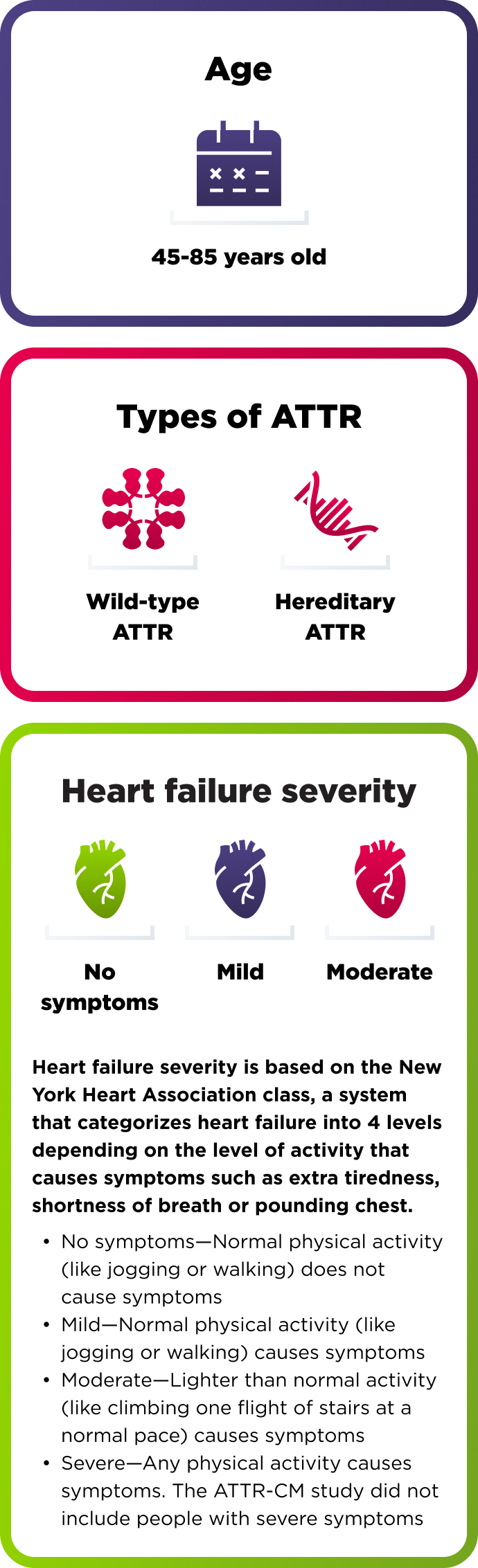
654 adults with cardiomyopathy of wild-type or hereditary ATTR
Up to 3½ years of data
The hATTR-PN study
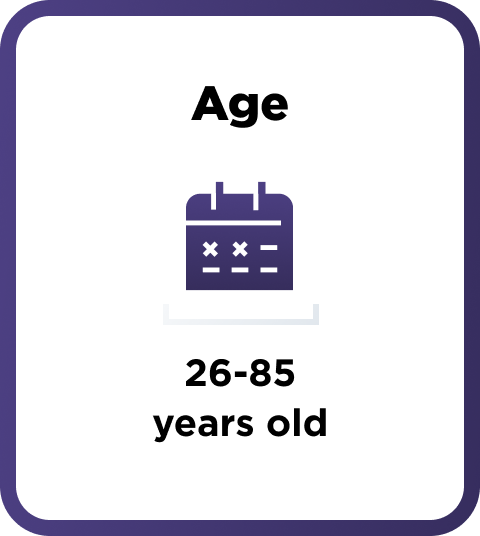
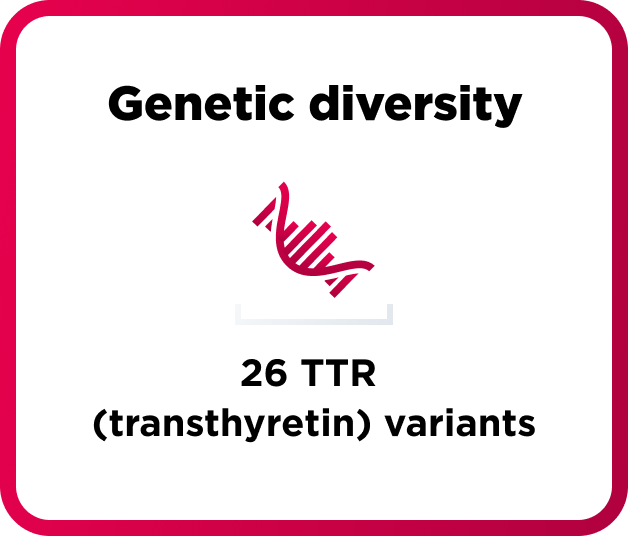
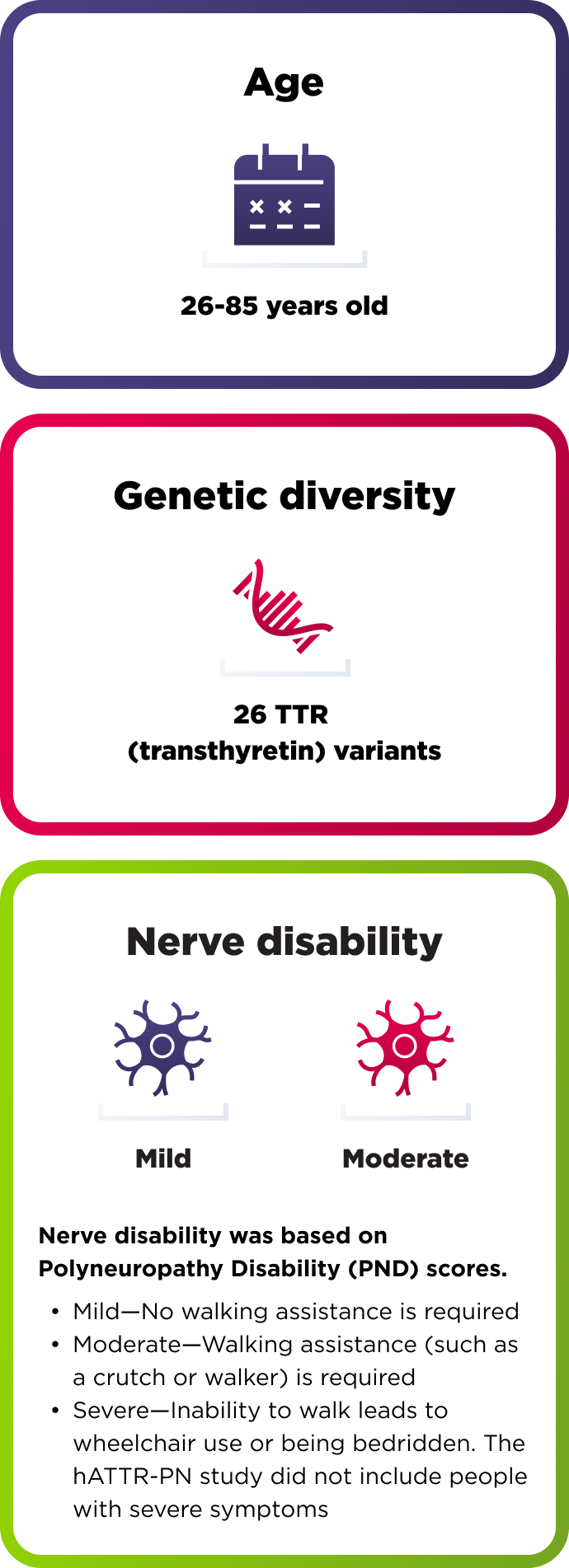
164 adults with polyneuropathy of hereditary ATTR
18 months of data
How AMVUTTRA was studied in people with ATTR-CM
A range of people with ATTR-CM took part in the study

How AMVUTTRA was studied in people with hATTR-PN
A range of people with hATTR-PN took part in the study
What are the most important things I should know about AMVUTTRA® (vutrisiran)?
AMVUTTRA can cause low vitamin A levels
Treatment with AMVUTTRA lowers the amount of vitamin A in your blood. Your doctor will tell you to take a vitamin A supplement every day. You should not take more than the amount of vitamin A recommended by your doctor.
Low vitamin A levels can affect vision. If you have problems with your vision (e.g., night blindness) while taking AMVUTTRA, talk to your doctor. Your doctor may refer you to an eye specialist.
What are the common side effects of AMVUTTRA?
The most common side effects of AMVUTTRA were pain in the arms or legs, pain in the joints, shortness of breath, and low vitamin A levels.
These are not all the possible side effects of AMVUTTRA. Talk to your doctor about side effects that you experience. You are encouraged to report negative side effects of prescription drugs to the U.S. Food and Drug Administration (FDA). Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
For additional information about AMVUTTRA, please see the full Prescribing Information.
What is AMVUTTRA?
AMVUTTRA is a prescription medicine that treats the:
Important Safety Information and Indications
What are the most important things I should know about AMVUTTRA® (vutrisiran)?
AMVUTTRA can cause low vitamin A levels
Treatment with AMVUTTRA lowers the amount of vitamin A in your blood. Your doctor will tell you to take a vitamin A supplement every day. You should not take more than the amount of vitamin A recommended by your doctor.
Low vitamin A levels can affect vision. If you have problems with your vision (e.g., night blindness) while taking AMVUTTRA, talk to your doctor. Your doctor may refer you to an eye specialist.
What are the common side effects of AMVUTTRA?
The most common side effects of AMVUTTRA were pain in the arms or legs, pain in the joints, shortness of breath, and low vitamin A levels.
These are not all the possible side effects of AMVUTTRA. Talk to your doctor about side effects that you experience. You are encouraged to report negative side effects of prescription drugs to the U.S. Food and Drug Administration (FDA). Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
For additional information about AMVUTTRA, please see the full Prescribing Information.
What is AMVUTTRA?
AMVUTTRA is a prescription medicine that treats the:
cardiomyopathy of
ATTR amyloidosis
polyneuropathy of hereditary
ATTR amyloidosis
transthyretin
transthyretin-mediated
amyloidosis
Kansas City Cardiomyopathy
Questionnaire
6-minute walk test
modified Neuropathy
Impairment Score +7
Quality of Life-Diabetic
Neuropathy